Ever wondered how the zinc plating process enhances the durability of metal components? It’s through a meticulous method of applying a zinc coating that provides corrosion resistance.
With years of experience in the metal fabrication industry, this insight is crafted to guide and inform.
The zinc plating process is an indispensable technique in the metal fabrication field. It not only prolongs the life of metal parts but also ensures they meet stringent industry standards for both protection and finish.
This article will outline the 5 key steps that are essential to achieving a high-quality zinc plating finish.
So keep reading to learn more!
Step#1 Pre-Treatment Stages
The initial phase of zinc plating involves key pre-treatment steps. According to Data Library Research, the global zinc plating market is expected to reach US$ 3.94 billion by 2030, growing at a CAGR of 6.9%. Here are the primary actions taken during the pre-treatment stage:
- Surface Degreasing: First, the metal surfaces are thoroughly degreased to strip away all contaminants like grease, oil, and dirt. This usually involves either a hot alkaline detergent bath or an electrocleaning process to achieve a chemically clean surface.
- Water Rinsing: Next, the components are rigorously rinsed with water to remove any leftover debris and cleaning solution. A proper rinse ensures that no contaminants interfere with the plating process.
- Acid Pickling: The metal is then immersed in an acidic solution in a process called pickling, which aims to remove any rust, scales, and surface oxides. This step is vital for a clean surface that will allow uniform plating.
- Fluxing: Finally, the pieces are fluxed by dipping them into a solution that helps to prevent oxidation during the drying process and immediately before the actual zinc plating. Fluxing provides a protective barrier against air and moisture, which could cause issues during plating.
Step#2 Bath Preparation
Following the meticulous pre-treatment, attention shifts to preparing the zinc plating bath, a critical component of the zinc plating process. After ensuring the metal surfaces are fully prepped, here are the crucial steps for bath preparation:
- Solution Mixing: Initially, a zinc plating solution is created by mixing water with various chemicals, including zinc oxide, acids, and other proprietary additives. The precise composition of this solution is essential to achieve the desired plating characteristics.
- Temperature Setting: The temperature of the plating bath is then carefully adjusted. The correct temperature is crucial for the efficiency of the plating process and the quality of the final coating.
- PH Balancing: At Zemetal, the pH level of the solution is meticulously controlled. A balanced pH ensures proper adhesion and uniformity of the zinc coating, which is critical for corrosion resistance.
- Bath Agitation: Lastly, the solution is agitated to maintain a homogeneous mixture. Continuous agitation prevents the settling of particulates and ensures that the zinc ions are evenly distributed throughout the bath for consistent plating results.
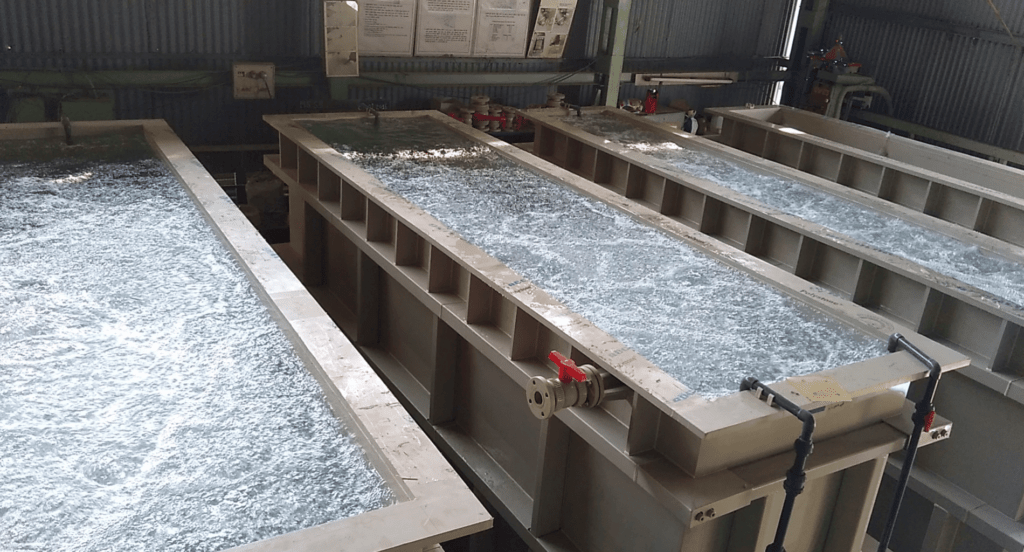
Step#3 Cathode and Anode Setup
With the bath preparation complete, the focus in the zinc plating process turns to the arrangement of cathodes and anodes, the pivotal elements for electroplating. Here are the steps taken to ensure the cathode and anode setup is ready for the plating process:
- Anode Installation: Zinc anodes are carefully positioned in the plating bath. These anodes often consist of high-purity zinc to ensure a consistent and clean source of zinc ions for the plating process.
- Cathode Positioning: The metal parts to be plated, which act as the cathodes, are then mounted on racks or barrels. They are strategically arranged to ensure even exposure to the zinc ions during the plating process. For instance, this careful arrangement is essential for achieving a uniform coating on all parts, which is critical for both the aesthetic and functional quality of the plated items.
- Electrical Connections: Electrical leads are securely attached to both the anodes and cathodes. It is critical to establish a good electrical connection to ensure that the current is evenly distributed across the surfaces to be plated.
- Current Adjustment: Finally, the power supply is turned on, and the current is adjusted to the appropriate level. The amperage is set according to the size of the parts and the required thickness of the zinc plating, which directly affects the deposition rate.
Step#4 Applying Electrical Current
Once the cathode and anode setup is complete, the next critical phase in the zinc plating process involves the application of electrical current. Here are the fundamental actions executed during the application of electrical current:
- Power Source Activation: The first action is to activate the rectifier or power source, which converts AC to DC current, necessary for the electroplating process to commence.
- Current Regulation: The direct current is then carefully regulated to the desired amperage based on the part size and the required zinc deposit thickness. Precise control of the current ensures uniform zinc plating.
- Time Monitoring: The duration for which the current is applied is strictly monitored. The plating time affects the thickness of the zinc layer; thus, timing is adjusted to achieve the specified coating requirements.
- Electrolysis Process: As the current flows, electrolysis occurs, causing zinc ions from the anodes to dissolve into the solution and deposit onto the cathodic metal parts. This electrochemical reaction is the core of the zinc plating process.
Step#5 Monitoring
With the electrical current diligently applied, the final and ongoing step in the zinc plating process is monitoring. Here are the essential actions involved in the monitoring stage:
- Visual Inspection: Regular visual inspections are conducted to check for any irregularities in the plating, such as bubbles, discoloration, or uneven areas, indicating potential issues with the plating process.

- Solution Analysis: The chemical composition of the plating bath is periodically analyzed to confirm the concentration of zinc and other chemicals remains within the optimal range for plating.
- Adjustments and Documentation: If any deviations from the expected results are detected during monitoring, adjustments are made to the process parameters. All findings and changes are meticulously documented for quality control and process improvement.
Step#6 Rinsing and Drying
After the careful monitoring of the electroplating process, the zinc-coated parts must be rinsed and dried to finalize the plating. Here are the precise actions taken during the rinsing and drying stage:
- Initial Rinsing: The plated parts are first removed from the plating bath and undergo an initial rinse to remove any adhering plating solution. This rinse, typically done with water, is crucial for washing away excess zinc ions and other chemicals. For instance, effectively removing these residues ensures the final product’s surface is clean and free from potential corrosion or quality defects.
- Counterflow Rinsing: At Zemetal, following the initial rinse, a counterflow rinsing technique is often employed, where the cleanest water is used in the final rinse stage and then cascades to the initial rinse. This method conserves water and ensures the parts are thoroughly clean.
- Hot Water Rinse: After counterflow rinsing, a hot water rinse may be applied to the parts. The elevated temperature aids in the evaporation of water and removal of any remaining residues.
- Drying: Finally, the parts are dried using one of several methods, such as air blowing, centrifuging, or oven drying. Drying must be complete to prevent water spots and to ensure that no moisture is trapped that could lead to corrosion.
Step#7 Quality Inspection and Testing
Following the rinsing and drying phase, the zinc-plated parts proceed to quality inspection and testing, which is crucial for ensuring that the plating process has met all specified standards and requirements. Here are the key procedures involved in the quality inspection and testing stage:
- Visual and Dimensional Inspection: Each part is visually inspected for surface defects, uniformity of the plating, and overall appearance. Dimensional inspection is also conducted to verify that the plating process has not affected the part’s dimensions beyond acceptable tolerances.
- Adhesion Test: To assess the adhesion quality of the zinc plating to the substrate, a tape test or bend test may be performed. These tests help in determining if the zinc coating is properly bonded to the base metal.
- Thickness Measurement: The thickness of the zinc layer is measured using a variety of methods, such as magnetic gauges or X-ray fluorescence (XRF). This ensures that the plating meets the specified thickness criteria.
- Salt Spray Test: According to American Galvanizers Association, a salt spray or corrosion test is often conducted to estimate the coating’s resistance to corrosion. This test involves exposing the plated parts to a saline mist environment for a specified period and evaluating the performance against corrosion standards.
This table outlines the key elements of the salt spray test for evaluating coating resistance to corrosion, as per the guidelines of the American Galvanizers Association.
| Test Component | Description | Purpose in Corrosion Assessment |
| Saline Mist Environment | Simulates harsh, corrosive conditions | Tests durability of coatings in extreme conditions |
| Duration of Exposure | Varies to assess long-term effects | Measures how long the coating can resist corrosion |
| Evaluation Against Standards | Comparing results with corrosion resistance norms | Ensures compliance with industry standards |
| Visual Inspection Post-Test | Checking for signs of rust or degradation | Determines physical impact of corrosion |
| Performance Metrics | Quantitative assessment of corrosion resistance | Provides objective data for quality assurance |
Conclusion
Reflecting on the zinc plating process, one can appreciate the blend of science and skill that goes into protecting and enhancing metal components. Every step from surface preparation to the final stage is crucial in ensuring that the end product can withstand the test of time and harsh environments.
For businesses looking to leverage the benefits of zinc plating, Zemetal offers comprehensive metal fabrication services tailored to your specific requirements. Contact us today.
Dive Deeper Into Our Resources
Looking for more diverse service options? Browse through our handpicked selections:
For some insightful reads, we’ve curated a list of recommended articles just for you:
Still haven’t found what you’re looking for? Don’t hesitate to contact us. We’re available around the clock to assist you.